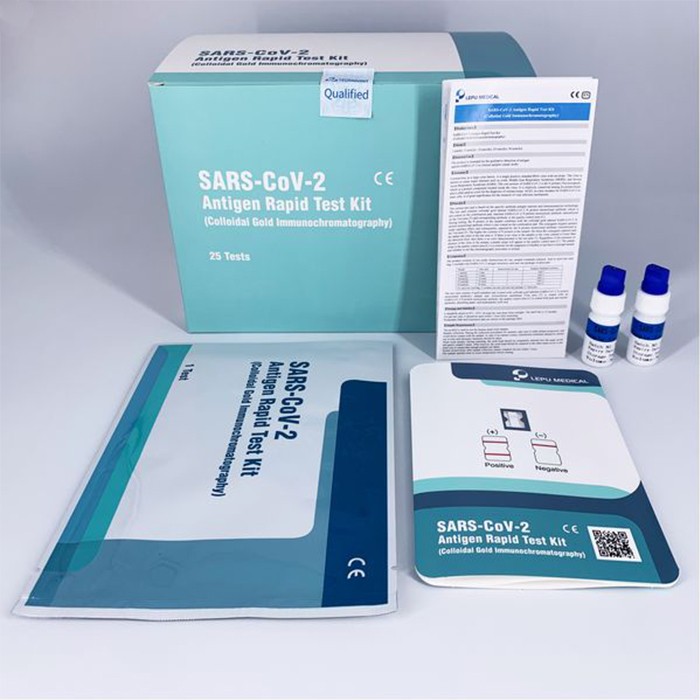
The product is intended for the qualitative detection of antigen against SARS-CoV-2 in clinical samples (nasal swab). The test has two central advantages: the swab only needs to be inserted into the front area of the nasal cavity and does not require a throat smear – which is why the test can be carried out by everyone conveniently. The current test card is based on the specific antibody-antigen reaction and immunoanalysis technology.
The product is intended for the qualitative detection of antigen against SARS-CoV-2 in clinical samples (nasal swab). The test has two central advantages: the swab only needs to be inserted into the front area of the nasal cavity and does not require a throat smear – which is why the test can be carried out by everyone conveniently. The current test card is based on the specific antibody-antigen reaction and immunoanalysis technology.
Announcement by the Austrian Federal Ministry of Education, Science & Research: “Antigen self-tests for all students – result in just 15 minutes”
Quality confirmed by the Paul Ehrlich Institute:
Comparative evaluation of the sensitivity of SARS-CoV-2 rapid antigen tests by the Paul Ehrlich Institute (PEI)
The results of Lepu Medical’s SARS-CoV-2 Antigen Rapid Test are officially recognized in 27 EU Countries: Official recommendation of the EU Commission